1.�������
TIC-600��9001cc��ɳ�Գ�Ϊ������ȫ���з���ȫ�ܻ�ͨ���͡�����������ģʽ������ɫ�������������ڼ��ͨ�����������ӡ�����ˮ��������Ʒ�������Ρ��л��ᡢ������������ӵ���ձ顢���ơ��Ƚ���Ӧ�ý����������ͬʱΪ�û������Զ��������Ի�������Ӧ�������������ձ�Ӧ�������Ρ�ʳ�������ũҵ����������ӵ�������ҵ�г������������ӵļ�����������

ͼ1. TIC-600����ɫ�������ͼ
2.����ɫ����ˮ�ʼ���е�Ӧ��
�����Ρ��������Ρ������Ρ�������������������dz�����������Ʒ�����������ˮ����ҵ��ˮ����ҽ�Ʒ�ˮ����ҪͶ�Ӹ������������������Եִ��ŷźͻ��ñ�����������ˮ���ر�ˮ���徲ʹ�����DZ�ڵ�Σ��������������ˮ�����Ⱦ��������õ���������Һ�ȡ��������ȡ��������ơ������ߡ��Ȱ��ͳ��������
�����κ���������������Ҫ�������������������ȵ���������Ʒ�����䶾��ѧӰ��ϴ������������������������Ѫ��Ѫ��ͱ���Ѫ���Ѱ�Ѫ����������Ѫ���״���ص�������������̥��С�������½�������Ϊ���û�����ϸ�����½������������������Ѫ�ܺͺ������ж����״����Ѫ�������֮һ�����䶾�Իή�;��ӵ���Ŀ�ͻ������������Ƥ���Ӵ�����������غ������ᵼ�º���ϵͳ���������ķ������桢��к��Ƥ��������֢״��������ʱ�᷺����Ѫ����ή������֢���鴤������ֱ��������Ч˥�߶���������ýӴ����������������ʳ�����������½����������շ���֢�����Գ��˵�������Ϊ 12 g����ͯΪ 5 g��Ӥ��Ϊ 1
g�����ֲ������ClO3-������ֲ��ϸ���� NO3-�����պ�������������ֲ��ȱ����������Ӱ��ֲ�����������Ӫ������ֳ���������
��������Ҫ���Գ�����Ϊ���������óͷ������ˮ�������ĸ���Ʒ�������ԭˮ�к��� Br-, Br-�����ͻᱻ��������ֱ������������������������ͨ��-OH ������������������������ο����ˮ�����������顢�㸹�顢������ȣ��������ʱ������˶������ƻ����������������������շ����鶯������ϸ���������������Ŵ����������������α����ʰ�֢�о�������Ϊ 2B ��(�ϸ��°�������)��DZ���°��������һ������ 70 kg �ij���������������ˮ 2 L������������Ũ��Ϊ45 ��g/L��0.5 ��g/L �� 0.05 ��g/L ʱ�����������°���Ϊ 10-4��10-5 �� 10-6�����°�Σ���ϴ������
�������ᣨDCAA�����������ᣨTCAA����Ҫ���Ȼ���������Ʒ��±������Ĵ��������TCAA�����»���������DCAA Ҳ���������α��������ֵ��徲ˮ����Ϊ�����°��������±�����ѱ�֤ʵ����ද�����°����»��䡢��ͻ�����������°�Σ��������������������Ʒ���ܺ��������о���ע����±������°�Σ��ռ��������Ʒ���°�Σ���� 91.9%���������
Ŀ������ˮ��������Խ��Խ��������������Ҳʱʱ����ˮ����Ⱦ��������ʹ���������ּ�����ˮ�ʼ�ء�����ˮ��Ӧ��Խ��Խ����ˮ�ʼ������������ˮ��Br-��BrO3-��ClO2-��ClO3-���������ᡢ��������������Ӻ����ļ�������ֱ��Ӱ������ˮˮ�������ѳ�Ϊˮ�ʼ���ͨ����Ŀ�����
����ɫ����TIC-600����м���͡�ȷ�ȸߡ��������á���������������ŵ��������ձ�Ӧ��������ˮ�������Ӻ����ļ�������
3.Ӧ��ʵ��
3.1 ����ɫ���ⶨˮ��Br-��ClO2-��ClO3-����������
3.1.1 �ο��� GB/T5750.10-2006����������ˮ��Br-��ClO2-��ClO3-�IJⶨ��
3.1.2 ���ù�ģ ��������������ˮ��ˮԴ���������Ρ������Ρ������ӵIJⶨ
3.1.3ʵ�鲿��
3.1.3.1�Լ�������
��ϴҺ��̼������[C(NaHCO3)=1.7mmol/l]-̼����[C(Na2CO3)=1.6mmol/l]����ȡ0.1428g̼�����ƺ�0.1908g̼�����������ڳ���ˮ��ϡ�͵�1L���������ҩ���Ż�ѧ�Լ�����˾��
�廯���Һ��1000��g/ml�����������α�Һ��1000��g/ml�������������α�Һ��1000��g/ml�������ұ��������ģ�
����ɫ���ǣ�TIC-600����9001cc��ɳ�Գ�Ϊ��������BS 224S������ƽ��������˹��ѧ����������������˾����0.1mg��������SHZ-D��ѭ��ˮʽ��ձã��������軪��������˾����������Һǹ���¹�����������10-100uL��100-1000uL��������0.22��m����Ĥ���Ϻ����ף������ܼ�����װ�ã�����ʵ��װ������˾��
��������ˮ��Ϊ����ˮ�������ʴ�18.25M����cm����������������ó���ˮϴ媲�����ס���������A-10����ˮ������������������
3.1.3.2 ʵ������
��ƷԤ���óͷ���
��ˮ����0.22��m����Ĥ���˳���������������Ӳ�ȸߵ�ˮ��������Ҫʱ�ɾ������ӽ�����֬�����پ�0.22��m����Ĥ���������Ժ��л���ĺ�ˮ�ȵر�ˮ���������Ⱦ�C18�����˳�ȥ�����
��Ʒ��Ԥ���óͷ���ע������ɫ���ǽ���ϵͳ������¼������������
��Һ���ƣ�
������ȡһ�������廯���Һ��1000��g/ml�����������α�Һ��1000��g/ml����
�����α�Һ��1000��g/ml����100ml��������ƿ�������ó�Ũ�����±�1��ʾϵ�л�����Һ�����
��1.������ҺŨ��
|
������
|
��ҺŨ�ȣ���g/l��
|
|
Std1
|
Std2
|
Std3
|
Std4
|
|
Br-
|
50
|
100
|
200
|
400
|
|
ClO2-
|
50
|
100
|
200
|
400
|
|
ClO3-
|
50
|
100
|
200
|
400
|
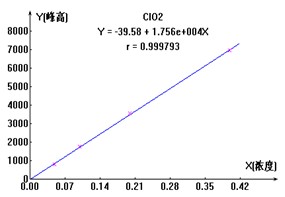
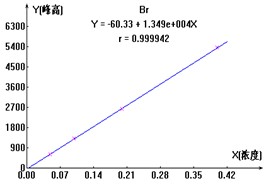
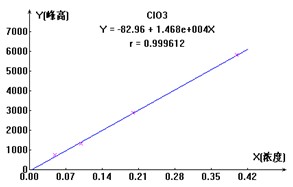
ͼ2. ClO2-��Br-��ClO3-���������ӱ�����ͼ
3.1.3.3ʵ������
1. ��Ʒ�ӱ������
������ˮ��Ʒ���ּӱ���Ũ������Ȼ��ÿ����Ʒ����Ʒ��Һ�����ⶨ����ƾ֤����Ч������ӱ������������ϸ���ݼ���2����3�����
��2.����ˮ��Ʒ�ӱ����������
|
������
|
������/��g/ml
|
|
����ˮ
|
�ӱ�50 ��g/l
|
������/%
|
�ӱ�100 ��g/l
|
������/%
|
�ӱ�200 ��g/l
|
������/%
|
|
ClO2-
|
0
|
53.4
|
106.8
|
100.9
|
100.9
|
193.8
|
96.9
|
|
Br-
|
68.6
|
118.4
|
99.7
|
169.4
|
100.8
|
274.1
|
102.8
|
|
ClO3-
|
30.9
|
80.0
|
98.2
|
133.8
|
102.9
|
234.7
|
101.9
|
��3.��ˮ��Ʒ�ӱ����������
|
������
|
������/��g/ml
|
|
����ˮ
|
�ӱ�50 ��g/l
|
������/%
|
�ӱ�100 ��g/l
|
������/%
|
�ӱ�200 ��g/l
|
������/%
|
|
ClO2-
|
0
|
45.7
|
91.4
|
92.5
|
92.5
|
195.9
|
97.9
|
|
Br-
|
0
|
50.2
|
100.4
|
102.6
|
102.6
|
200.4
|
100.2
|
|
ClO3-
|
126.9
|
177.8
|
101.8
|
224.2
|
97.3
|
328.7
|
100.9
|
3.1.4����
�����ݿ�֪��������ɫ������ˮ��Br-��ClO2-��ClO3-����������ȷ�ȸ�������С���Ũ�ȵ�����Ҫ����ӿ��������ȫ֪�㻷����ҵ����ˮ��Br-��ClO2-��ClO3-���������Ӽ������������
3.2 ����ɫ���ⶨˮ��BrO3-����
3.2.1�ο��� GB/T5750.10-2006����������ˮ�������εIJⶨ��
3.2.2 ���ù�ģ ��������������ˮ��ˮԴ���������Ρ������Ρ������ӵIJⶨ
3.2.3ʵ�鲿��
3.2.3.1 �Լ�������
��ϴҺ��̼������[C(NaHCO3)=1.7mmol/l]-̼����[C(Na2CO3)=1.6mmol/l]����ȡ0.1428g̼�����ƺ�0.1908g̼�����������ڳ���ˮ��ϡ�͵�1L���������ҩ���Ż�ѧ�Լ�����˾��
�����α�Һ��1000��g/ml�������ұ��������ģ�
����ɫ���ǣ�TIC-600����9001cc��ɳ�Գ�Ϊ��������BS
224S������ƽ��������˹��ѧ����������������˾����0.1mg��������SHZ-D��ѭ��ˮʽ��ձã��������軪��������˾����������Һǹ���¹�����������10-100uL��100-1000uL��������0.22��m����Ĥ���Ϻ����ף������ܼ�����װ�ã�����ʵ��װ������˾��
��������ˮ��Ϊ����ˮ�������ʴ�18.25M����cm����������������ó���ˮϴ媲�����ס���������A-10����ˮ������������������
3.2.3.2 ʵ������
��ƷԤ���óͷ���
��ˮ����0.22��m����Ĥ���˳���������������Ӳ�ȸߵ�ˮ��������Ҫʱ�ɾ������ӽ�����֬�����پ�0.22��m����Ĥ���������Ժ��л���ĺ�ˮ�ȵر�ˮ���������Ⱦ�C18�����˳�ȥ�����ˮ�������Ӷ�BrO3-���Ӳⶨ����Ӱ��������Ʒ�辭������ȡ��Ag���������óͷ��Գ�ȥCl���������
��Ʒ��Ԥ���óͷ���ע������ɫ���ǽ���ϵͳ������¼������������
��Һ���ƣ�
��ȡһ�����������α�Һ��1000��g/ml���������ó�Ũ��Ϊ10��g/ml�������δ���Һ�����ٻ�����ȡ0ml��0.1ml��0.2ml��0.5ml��1.0ml��10��g/ml�������δ���Һ��100ml��������ƿ�������ӳ���ˮ�����������Ƴ�0��g/l��10��g/l��20��g/l��50��g/l��100��g/l��ϵ�б���Һ��������ͼ3 ΪBrO3-���ӱ�����ͼ�����
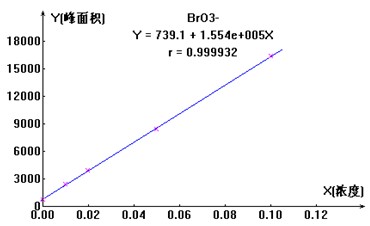
ͼ3. BrO3-���ӱ�����ͼ
3.2.3.3ʵ������
1.��Ʒ�ӱ������
������ˮ��Ʒ����ˮ��Ʒ���ּӱ���Ũ������Ȼ��ÿ����Ʒ����Ʒ��Һ�����ⶨ����Ȼ��ƾ֤����Ч������ӱ������������ϸ���ݼ���4�����
��4. ����ˮ����ˮ��Ʒ�ӱ����������
|
��Ʒ
|
BrO3-������/��g/l
|
|
δ�ӱ�
|
�ӱ�10 ��g/l
|
������/%
|
�ӱ�20 ��g/l
|
������/%
|
�ӱ�40 ��g/l
|
������/%
|
|
����ˮ
|
1.1
|
10.8
|
97.0
|
20.48
|
96.9
|
38.7
|
96.8
|
|
��ˮ
|
0
|
10.41
|
104.1
|
21.33
|
106.7
|
38.8
|
97.0
|
3.2.4����
��ʵ�����ݿ�֪��������ɫ������ˮ��BrO3-������ȷ�ȸ��������Ũ�ȵ�����Ҫ����ӿ��������ȫ֪�㻷����ҵ����ˮ��BrO3-���Ӽ������������
3.3 ����ɫ���ⶨˮ�ж������ᡢ��������
3.3.1�ο��� GB/T5749-2006����������ˮ��������
3.3.2 ���ù�ģ ��������������ˮ��ˮԴ�ж������ᡢ��������IJⶨ
3.3.3ʵ�鲿��
3.3.3.1 �Լ�������
��ϴҺ��̼������[C(NaHCO3)=3mmol/l]-̼����[C(Na2CO3)=3mmol/l]����ȡ0.252g̼�����ƺ�0.3018g̼�����������ڳ���ˮ��ϡ�͵�1L���������ҩ���Ż�ѧ�Լ�����˾���ң�
�������ᡢ���������Һ��1000��g/ml�������ұ��������ģ�
����ɫ���ǣ�TIC-600����9001cc��ɳ�Գ�Ϊ��������BS
224S������ƽ��������˹��ѧ����������������˾����0.1mg��������SHZ-D��ѭ��ˮʽ��ձã��������軪��������˾����������Һǹ���¹�����������10-100uL��100-1000uL��������0.22��m����Ĥ���Ϻ����ף������ܼ�����װ�ã�����ʵ��װ������˾��
��������ˮ��Ϊ����ˮ�������ʴ�18.25M����cm����������������ó���ˮϴ媲�����ס���������A-10����ˮ������������������
3.3.3.2 ʵ������
��ƷԤ���óͷ���
��ˮ����0.22��m����Ĥ���˳���������������Ӳ�ȸߵ�ˮ��������Ҫʱ�ɾ������ӽ�����֬�����پ�0.22��m����Ĥ���������Ժ��л���ĺ�ˮ�ȵر�ˮ���������Ⱦ�C18�����˳�ȥ�������ˮ�������ӶԶ����������Ӳⶨ����Ӱ��������Ʒ�辭������ȡ��Ag���������óͷ��Գ�ȥCl���������
��Ʒ��Ԥ���óͷ���ע������ɫ���ǽ���ϵͳ������¼������������
��Һ���ƣ�
��ȡһ�����Ķ��������Һ��1000��g/ml�������������Һ��1000��g/ml���������óɵĶ������ᣨŨ��Ϊ10��g/ml�����������ᣨŨ��Ϊ5.0��g/ml����������Һ�����ٻ�����ȡ0ml��1.0ml��2.0ml��5.0ml��10.0ml��10��g/ml�Ķ������ᡢ���������������Һ��100ml��������ƿ�������ӳ���ˮ�����������Ƴɶ������ᡢ��������ϵ�л�������Һ��������ͼ4 Ϊ�������ᡢ�����������ӱ�����ͼ�����
��5.�������ᡢ�������������ҺŨ��
|
������
|
��ҺŨ�ȣ���g/l��
|
|
Std1
|
Std2
|
Std3
|
Std4
|
|
��������
|
50
|
100
|
200
|
500
|
|
��������
|
100
|
200
|
400
|
1000
|
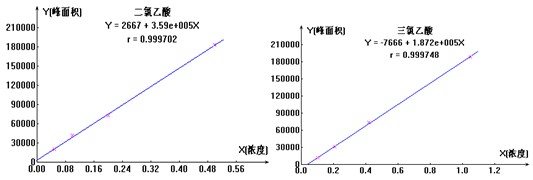
ͼ4. �������ᡢ�������������ͼ
3.3.3.3ʵ������
1.��Ʒ�ӱ������
������ˮ��Ʒ����ˮ��Ʒ���ּӱ���Ũ�ȶ������ᡢ������������Ȼ��ÿ����Ʒ����Ʒ��Һ�����ⶨ����Ȼ��ƾ֤����Ч������ӱ������������ϸ���ݼ���6�����
��6. ����ˮ����ˮ��Ʒ�ӱ����������
|
��Ʒ
|
�������������/��g/l
|
|
δ�ӱ�
|
�ӱ�100 ��g/l
|
������/%
|
�ӱ�200 ��g/l
|
������/%
|
�ӱ�500��g/l
|
������/%
|
|
����ˮ
|
0
|
91.3
|
91.3
|
196.3
|
98.2
|
486.2
|
97.3
|
|
��ˮ
|
0
|
94.2
|
94.2
|
192.1
|
96.1
|
503.2
|
100.6
|
|
��Ʒ
|
�������������/��g/l
|
|
δ�ӱ�
|
�ӱ�100 ��g/l
|
������/%
|
�ӱ�200��g/l
|
������/%
|
�ӱ�500��g/l
|
������/%
|
|
����ˮ
|
0
|
96.3
|
96.3
|
188.3
|
94.2
|
478.62
|
95.6
|
|
��ˮ
|
0
|
93.8
|
93.8
|
196.7
|
98.4
|
482.1
|
96.4
|
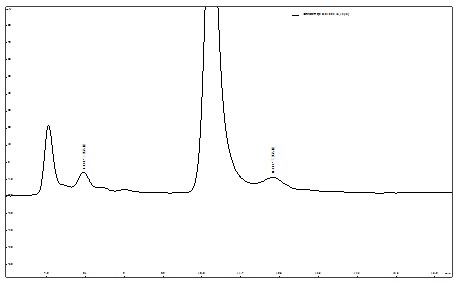
ͼ5. ����ˮ��Ʒ�������ᡢ��������ӱ���ͼ
3.3.4����
��ʵ�����ݿ�֪��������ɫ������ˮ�ж������ᡢ��������ȷ�ȸ��������Ũ�ȵ�����Ҫ����ӿ��������ȫ֪�㻷����ҵ����ˮ���ж������ᡢ��������������������

